 No matter where you are in the world; whether it’s Africa or Antarctica, Borneo or Brazil, the desert or the rain forest, a cruise ship or a Navy cruiser; The STAT PATH Point of Care Potassium Blood Analyzer is designed to give an accurate result within 40 seconds using only 1 or 2 drops of blood.
No matter where you are in the world; whether it’s Africa or Antarctica, Borneo or Brazil, the desert or the rain forest, a cruise ship or a Navy cruiser; The STAT PATH Point of Care Potassium Blood Analyzer is designed to give an accurate result within 40 seconds using only 1 or 2 drops of blood.
For patients who need to test their potassium level on a regular basis, STAT PATH’s POC Blood Analyzer is both cost and time effective.
Our unique system puts critical patient “stat” testing in the hands of caregivers; quickly, efficiently and inexpensively. With STAT PATH’s Point of Care Analyzers (POC) and disposable Cartridges, a technician or a patient can quickly test a potassium level during dialysis to monitor and adjust the treatment.
Call us today at 1 888 930 9932 EST
or leave us a message at the bottom of this page.
STAT PATH’s Point of Care blood analyzers improve the quality and reduce the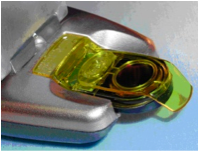 cost of health care by providing an in vitro diagnostic product that facilitates early diagnosis at the point of care (POC).
cost of health care by providing an in vitro diagnostic product that facilitates early diagnosis at the point of care (POC).
Point of Care in vitro diagnostic testing refers to the performance of tests at the patient’s site that are identical to standard tests processed by central laboratories. STAT PATH’S Point of Care Blood Analyzer is designed to be so simple to use that a layperson can obtain the same degree of accuracy and reliability as trained personnel.
Here’s where we were at the beginning of April 2016.
STAT PATH’s Point of Care Blood Analyzer has been granted 510(k) clearance from the FDA.
STAT PAT H’S Point of Care’s products are the first truly portable POC systems for most critical care analytes as the cartridges require no refrigeration and the device is palm-sized.
H’S Point of Care’s products are the first truly portable POC systems for most critical care analytes as the cartridges require no refrigeration and the device is palm-sized.
Unlike existing technologies, STAT PATH’s products can serve all medical markets, including hospitals, physician offices, mobile hospital units, ambulances, and patients’ homes.
In the era of Managed Care, the hospital central laboratory has become a cost center. Several national hospital chains have divested laboratory management and test services to outside laboratory enterprises and thus have realized significant cost reductions. The hospitals still need to maintain expensive and labor-intensive “stat” (or immediate) satellite laboratories. Thus any test system simple and cost effective enough to move testing from the stat laboratory to the Point of Care, and in so doing streamlining the testing process, is economically and clinically very attractive. For physician offices, ambulances, mobile hospital units, the military and home health care this technology represents a true paradigm shift allowing real-time diagnostic assessment and treatment changes for all medical venues, and without access to trained laboratory personnel and expensive, difficult to maintain equipment. Previous devices have claimed to be portable but are not truly portable as the cartridges must be refrigerated, the price is substantially higher, and the level of training required to use them is significant. Despite these drawbacks, there has been market acceptance of these devi ces and they thus serve as a transitional technology to STAT PATH’s fully functional, POC system.
ces and they thus serve as a transitional technology to STAT PATH’s fully functional, POC system.
STAT PATH’s technological developments expand the POC venue beyond the hospital, to physician offices, out-patient sites, emergency vehicles and, the patient’s home. To penetrate markets beyond the hospital venue, POC products must achieve levels of reliability and simplicity sufficient to be designated CLIA Waived. CLIA Waiver (see below), is a designation granted by the FDA to products that have two key attributes, both of which are inherent in STAT PATH’S technology:
Waived POC disposable products must guarantee single use. (The onboard cartridge computer chip erases after calibration, eliminating any chance of second use).
An untrained user operating such devices, such as a patient in his home, must be able to obtain the same results as a trained user. (A device can only be operated one way, no matter what the level of sophistication of the operator might be: insert cartridge, calibrate the cartridge, peel cap when prompted by the analyzer icon, and apply 40 to 60 micro liters of blood which is 1 to 3 drops).
In addition to the simplicity and reliability inherent in CLIA Waived Status, to successfully penetrate vertical markets beyond the hospital venue, a POC product should be saleable through the standard supply distribution chain. This requires long ambient temperature shelf life (impossible with today’s liquid-based systems), and low-cost manufacturing to ensure sufficient profit margin. Both these attributes are inherent in STAT PATH’s technology and products. Currently there are no competitive devices CLIA waived.
Potential venues and  customers for STAT PATH’s products, nationally and internationally, include hospitals, physician offices, outpatient facilities (including surgi-centers and dialysis units), patient homes, ambulances, the military, disaster preparedness organizations, and veterinary medicine facilities.
customers for STAT PATH’s products, nationally and internationally, include hospitals, physician offices, outpatient facilities (including surgi-centers and dialysis units), patient homes, ambulances, the military, disaster preparedness organizations, and veterinary medicine facilities.
UPCOMING EVENTS
The analyzers will allow for testing for most of the common central laboratory tests at the POC, with results available in 40 seconds while only using 2–3 drops of blood. Tests will include:
-
- Potassium
- Sodium
- Calcium
- Lithium
- Electrolytes
- Chem 8
Click below for more info on the need for our blood analyzer.
Leave Us A Message!

2 thoughts on “Home Page Stat Path Technologies”